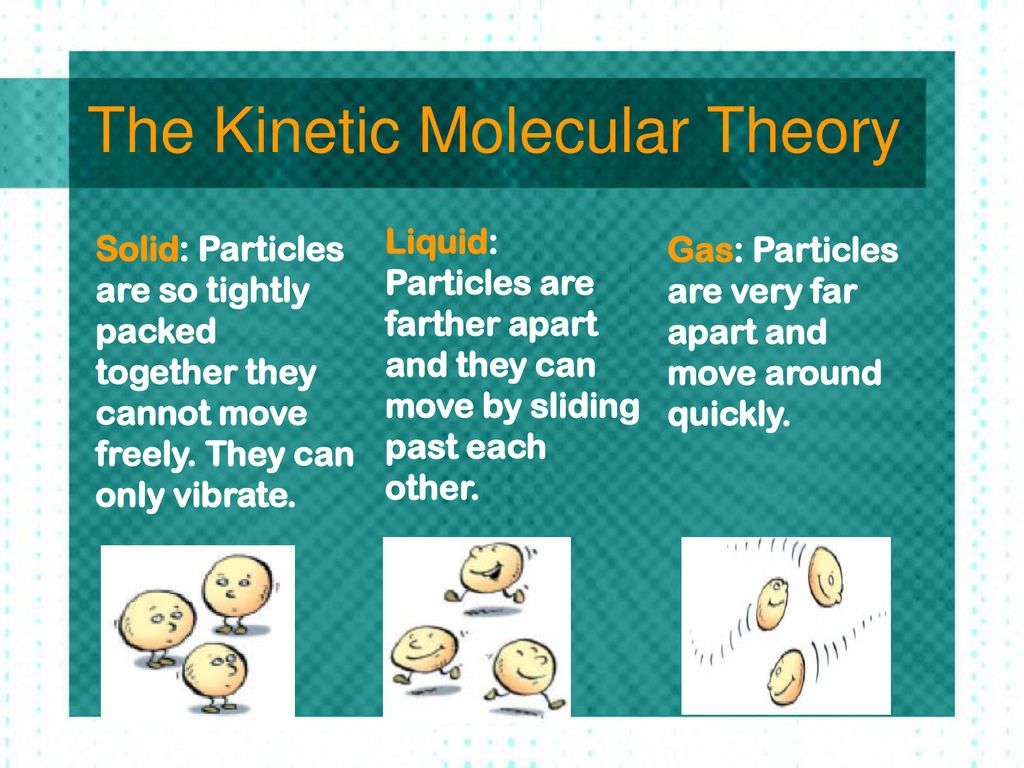
What Are The 5 Components Of Kinetic Molecular Theory . Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. this theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual chemical species that. Gases consist of very large numbers of tiny spherical particles that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. A gas is composed of molecules that are.
from slideplayer.com
A gas is composed of molecules that are. this theory is based on the following five postulates described here. Gases consist of very large numbers of tiny spherical particles that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. The term “molecule” will be used to refer to the individual chemical species that. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions.
States of Matter & Molecular Theory ppt download
What Are The 5 Components Of Kinetic Molecular Theory Gases consist of very large numbers of tiny spherical particles that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. The term “molecule” will be used to refer to the individual chemical species that. Gases consist of very large numbers of tiny spherical particles that are. this theory is based on the following five postulates described here. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. A gas is composed of molecules that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of.
From studyfinder.org
A Comprehensive Guide to Understanding the Pogil Molecular What Are The 5 Components Of Kinetic Molecular Theory Gases consist of very large numbers of tiny spherical particles that are. A gas is composed of molecules that are. The term “molecule” will be used to refer to the individual chemical species that. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. this. What Are The 5 Components Of Kinetic Molecular Theory.
From dxonwlofz.blob.core.windows.net
What Is Molecular Theory Matter at Marie Craven blog What Are The 5 Components Of Kinetic Molecular Theory Gases consist of very large numbers of tiny spherical particles that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. this theory is based on the following five postulates described here. The term “molecule” will be used to refer to the individual chemical species that.. What Are The 5 Components Of Kinetic Molecular Theory.
From slideplayer.com
States of Matter & Molecular Theory ppt download What Are The 5 Components Of Kinetic Molecular Theory the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. this theory is based on the following five postulates described here. Gases consist of very large numbers of tiny spherical particles that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains. What Are The 5 Components Of Kinetic Molecular Theory.
From www.slideserve.com
PPT Theory PowerPoint Presentation, free download What Are The 5 Components Of Kinetic Molecular Theory A gas is composed of molecules that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. this theory is based on the following five postulates described here. Gases consist of very large numbers of tiny spherical particles that are. The term “molecule” will be used. What Are The 5 Components Of Kinetic Molecular Theory.
From es.slideshare.net
Molecular Theory What Are The 5 Components Of Kinetic Molecular Theory Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. The term “molecule” will be used to refer to the individual chemical species that. the kinetic molecular theory (kmt) is a. What Are The 5 Components Of Kinetic Molecular Theory.
From slideplayer.com
MOLECULAR THEORY ppt download What Are The 5 Components Of Kinetic Molecular Theory the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. The term “molecule” will be used to refer to the individual chemical species that. Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. the kinetic molecular theory (kmt) is a. What Are The 5 Components Of Kinetic Molecular Theory.
From sciencenotes.org
Molecular Theory of Gases What Are The 5 Components Of Kinetic Molecular Theory Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. this theory is based on the following five postulates described here. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. The term “molecule” will be used to refer to the. What Are The 5 Components Of Kinetic Molecular Theory.
From slideplayer.com
The Molecular Theory Review Notes ppt download What Are The 5 Components Of Kinetic Molecular Theory the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. Gases consist of very large numbers of tiny spherical particles that are. this theory is based on the following five postulates described here. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains. What Are The 5 Components Of Kinetic Molecular Theory.
From www.slideserve.com
PPT Molecular Theory PowerPoint Presentation, free download What Are The 5 Components Of Kinetic Molecular Theory the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. A gas is composed of molecules that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. Kinetic molecular theory states that gas particles are. What Are The 5 Components Of Kinetic Molecular Theory.
From www.slideserve.com
PPT Molecular Theory PowerPoint Presentation, free download What Are The 5 Components Of Kinetic Molecular Theory the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. this theory is based on the following five postulates described here. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. A gas. What Are The 5 Components Of Kinetic Molecular Theory.
From www.chem.uwec.edu
molecular theory of matter What Are The 5 Components Of Kinetic Molecular Theory Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. A gas is composed of molecules that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains. What Are The 5 Components Of Kinetic Molecular Theory.
From rumble.com
molecular theory, ideal gases Chemistry What Are The 5 Components Of Kinetic Molecular Theory the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. Gases consist of very large numbers of tiny spherical particles that are. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. this. What Are The 5 Components Of Kinetic Molecular Theory.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free What Are The 5 Components Of Kinetic Molecular Theory A gas is composed of molecules that are. The term “molecule” will be used to refer to the individual chemical species that. Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. What Are The 5 Components Of Kinetic Molecular Theory.
From www.slideshare.net
Molecular Theory What Are The 5 Components Of Kinetic Molecular Theory this theory is based on the following five postulates described here. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. Gases consist. What Are The 5 Components Of Kinetic Molecular Theory.
From slideplayer.com
States of Matter & Molecular Theory ppt download What Are The 5 Components Of Kinetic Molecular Theory Gases consist of very large numbers of tiny spherical particles that are. Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. The term “molecule” will be used to refer to the individual chemical species that. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described. What Are The 5 Components Of Kinetic Molecular Theory.
From slideplayer.com
The Molecular Theory ppt download What Are The 5 Components Of Kinetic Molecular Theory the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. A gas is composed of molecules that are. Gases consist of very large numbers of tiny spherical particles that are. this theory is based on the following five postulates described here. the kinetic molecular. What Are The 5 Components Of Kinetic Molecular Theory.
From slideplayer.com
States of Matter & Molecular Theory ppt download What Are The 5 Components Of Kinetic Molecular Theory the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. The term “molecule” will be used to refer to the individual chemical species that.. What Are The 5 Components Of Kinetic Molecular Theory.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free What Are The 5 Components Of Kinetic Molecular Theory A gas is composed of molecules that are. The term “molecule” will be used to refer to the individual chemical species that. the kinetic molecular theory (kmt) is a simple microscopic model that effectively explains the gas laws described in previous modules of. Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions.. What Are The 5 Components Of Kinetic Molecular Theory.